Monarch larvae are specialist herbivores of plants in the family Asclepiadaceae (milkweeds), and have been recorded feeding on 27 different North American species in this family (Malcolm and Brower 1989). The larvae sequester toxic steroids, known as cardenolides, from milkweed (Brower 1969; Brower & Glazier 1975; Malcolm 1991, 1995), and they use these cardenolides as a defense against predators. The bad taste and toxicity of both the larvae and adults are advertised by conspicuous, warning coloration. When a bird or other predator tastes a monarch, it learns to associate this color pattern with the bad taste and avoids preying on monarchs in the future.
How do Chemicals in Milkweed Benefit Monarchs?
Early in the 20th century, Poulton (1909) suggested that the reason monarchs feed only on milkweeds might be because chemicals in the milkweeds provide them with some protection from predators. However, it was not until the 1960’s that researchers discovered that cardenolides were the chemicals in milkweed that made monarchs toxic and bitter-tasting (Parsons 1965). Since then, several studies have shown that monarchs are able to sequester these toxic compounds. For example, Figure 1, drawn from data collected by Malcolm and Brower (1989) shows the range of cardenolide concentrations found in one species of milkweed, Asclepias viridis (Green Antelopehorns), and adult monarchs that fed on that milkweed as larvae. Since the adults do not eat milkweed, this shows that they have stored the cardenolides that they ingested as larvae.
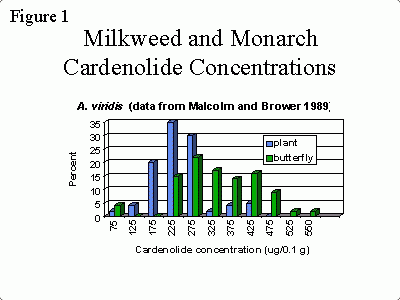
Frequency distribution of cardenolide concentrations in A. viridis (collected in Louisiana) and monarch butterflies fed this species. This shows that monarchs are able to store cardenolides that they ingest as larvae.
The cardenolides obtained from milkweed make monarchs toxic to many vertebrate predators (e.g. Brower et al. 1974, Rothschild et al. 1975). For example, captive bluejays fed monarchs containing cardenolides throw up after eating the monarchs, and the probability of their throwing up increases with increasing cardenolide concentration in the monarch (e.g. Brower 1969, Brower et al. 1972, Brower and Glazier 1975). Other work has shown that wild monarchs containing high levels of cardenolides are also less susceptible to natural predation by both birds (Fink and Brower 1981) and mice (Glendinning et al. 1988).
Protection from vertebrate predators is probably most important to later larval instars, pupae, and adults, whereas earlier instars are more likely to be eaten by arthropods such as mites, spiders, ants, wasps and bugs. Vertebrates tend to be long-lived and able to use learning to avoid noxious foods, and most people agree that the bright colors found on monarchs and other toxic insects help to identify them to vertebrate predators that have learned to associate these colors with bad tastes (e.g. Turner 1984). However, it is not clear that invertebrate predators learn to associate bright colors with bad taste, or even that monarchs are toxic to invertebrate predators.The lack of knowledge on the relationship between milkweed, monarchs, and invertebrate predators represents an important gap in our knowledge.
Based on reported MLMP data, most mortality of monarchs probably occurs in the early instars. As alluded to above, a portion of this mortality is due to invertebrate predation. Chrysoperla rufilabris (Green Lacewing) larvae is a common inhabitant of milkweed species and a voracious predator of monarch eggs. A 2007 Monarch Lab study determined that C. rufilabris larvae depleted 35 eggs from milkweed in 24 hours, seemingly unaffected by the monarch’s toxicity. Results of this study demonstrate the susceptibility of monarchs to predation by invertebrates and provide valuable information for future studies of invertebrate predation and cardenolide sensitivity (Monarchs in a Changing World: Biology and Conservation of an Iconic Insect).

_insect_eating_egg_mlmpweb_220_220_80_all_3_c1_200_200_all_3_c1.jpg)
_dead_monarch_cater_220_220_80_all_3_c1_200_200_all_3_c1.jpg)
_image032_220_220_80_all_3_c1_200_200_all_3_c1.jpg)
Variation Among Milkweeds and Monarchs in Toxin Concentration
Roeske et al. (1976) showed that different species of milkweed contained different concentrations of toxins, and that monarchs reared on these species also varied in the amount of toxins in their bodies. However, there is not a perfect correlation between the amount of cardenolides in plants, and the monarchs that consume them. Figure 3 shows the concentration of cardenolides in various milkweed species, and the concentrations in adult monarchs that were fed these milkweeds as larvae. Monarchs that were fed A. viridis from Florida had the highest cardenolide concentrations, even though the plants themselves had intermediate levels. It appears that monarchs fed high cardenolide plants do not concentrate the toxins as effectively as do monarchs from intermediate and low cardenolide plants (Malcolm and Brower 1989).

Mean cardenolide concentrations in 12 milkweed species, and monarchs fed these plants as larvae. Each point represents mean values from 18 to 158 pairs of plants and monarchs. (Photo: Data from Malcolm and Brower (1989))
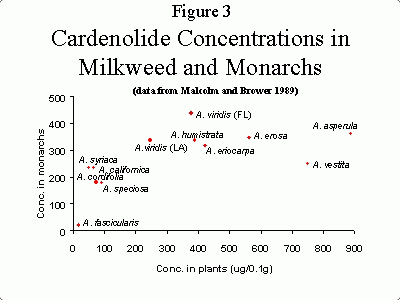
In addition to variation in cardenolide concentration between milkweed plants of different species, there is also a great deal of variation within plant species. For example, one of the plant species shown in figure 3, A. syriaca (common milkweed), had cardenolide concentrations ranging from 0 to 792 µg/0.1 g dry weight (Malcolm and Brower 1989). While it is not completely clear what causes so much variation, work by Steve Malcolm and his collaborators (e.g. Malcolm and Zalucki 1996) showed that plants produced more cardenolides within 24 hours after being damaged. This suggests that cardenolides are an example of an "inducible" plant defense; this would benefit the plant, since it would only invest in defenses when it needed them. Perhaps variation in plant cardenolide concentration is due partly to variation in damage by herbivores.
Research by Alfonso Alonso has shown that monarchs lose cardenolides as they get older. Over a period of 30 days the concentration of cardenolides in the wings and abdomens of monarchs kept in an outdoor cage decreased by about 600 µg/0.1 g dry weight (Alonso-Mejia and Brower 1994). Similar decreases in concentration occur over the overwintering period in Mexico (Alonso 1996), suggesting that monarchs become less protected from predators as they get older. Alonso thinks that this decrease is due to scale loss from the butterflies’ wings, denaturation of the cardenolides, and excretion (Alonso 1996).
Effects of Milkweed Defenses on Monarch Larvae
Monarchs are thought by many people to be specialists that incur little cost to feeding on milkweeds. In fact, they do appear to benefit both nutritionally and defensively from their milkweed diet (Malcolm 1991, 1995). However, plants use a variety of defenses against the animals that eat them, and the cardenolides in milkweeds are present to protect the plants, not to protect the monarchs eating them. The defensive system of milkweeds was well-described by Dussourd (1993): "milkweed leaves contain a ramifying network of latex canals pressurized with a lethal brew of toxic cardenolides in a quick-setting glue." Thus, it should not be surprising that monarchs do suffer some ill effects from feeding on milkweed.
Experimental evidence has shown that the larvae are negatively affected by the milky latex characteristic of milkweeds, which can gum up the mandibles of small larvae so that they can no longer eat. Zalucki & Brower (1992) conducted an experiment in which they followed almost 700 monarchs from the egg stage to the second instar stage. They found very low survival (from 3 to 11%), and determined that about 30% of larvae were killed when they became mired in milkweed latex. Supporting this result, Malcolm and Zalucki (1996) and Zalucki & Malcolm (1998) found higher survival of first instar larvae when they fed on leaves on which the latex flow had been cut off.
In addition to the negative effects of the latex, there is evidence that monarchs may also be affected negatively by milkweed cardenolides (Zalucki et al. 1990). Early instar survival is lower on plants with high cardenolide levels, and larvae that happened to swallow some of the latex had a strong response, as described by Zalucki & Brower (1992): "the anterior portion of the larva’s body looked pale, the larva backed onto the silk mat, assumed a position with its prolegs anchored, and raised the anterior part of its body and tucked its head down. The larva would remain cataleptic in this position for up to 10 minutes before continuing its biting behavior." Other evidence that high cardenolide levels may be harmful to larvae is the fact that females prefer to oviposit on plants that have intermediate levels of this toxin (Zalucki et al. 1990, Oyeyele and Zalucki 1990, Van Hook and Zalucki 1991).
Monarch larvae appear to use a variety of strategies to avoid these affects. Small larvae use a 'trenching' behavior, in which they chew a small circle through the surface of the leaf, making a circular area to which latex does not flow. Larger larvae cut through the mid-vein of a leaf, cutting off latex flow to the entire leaf. Both of these behaviors provide protection from the sticky latex, and possibly also from the toxins, which are more concentrated in the latex (Zalucki and Brower 1992).
_2nd_instar_Eating_220_220_80_all_3_c1_200_200_all_3_c1.JPG)


